How to Find Hybridization Around Central Atom
If the steric number is 3 the atom is mathrmsp2 hybridized. Steric number of OCl2 Number of bonded atoms attached to oxygen Lone pair on oxygen atom As per the OCl 2 lewis structure the oxygen central atom is bonded with two chlorine atoms and contains 2 lone pairs as well.

Aleks Identifying Hybridization In A Small Molecule Youtube
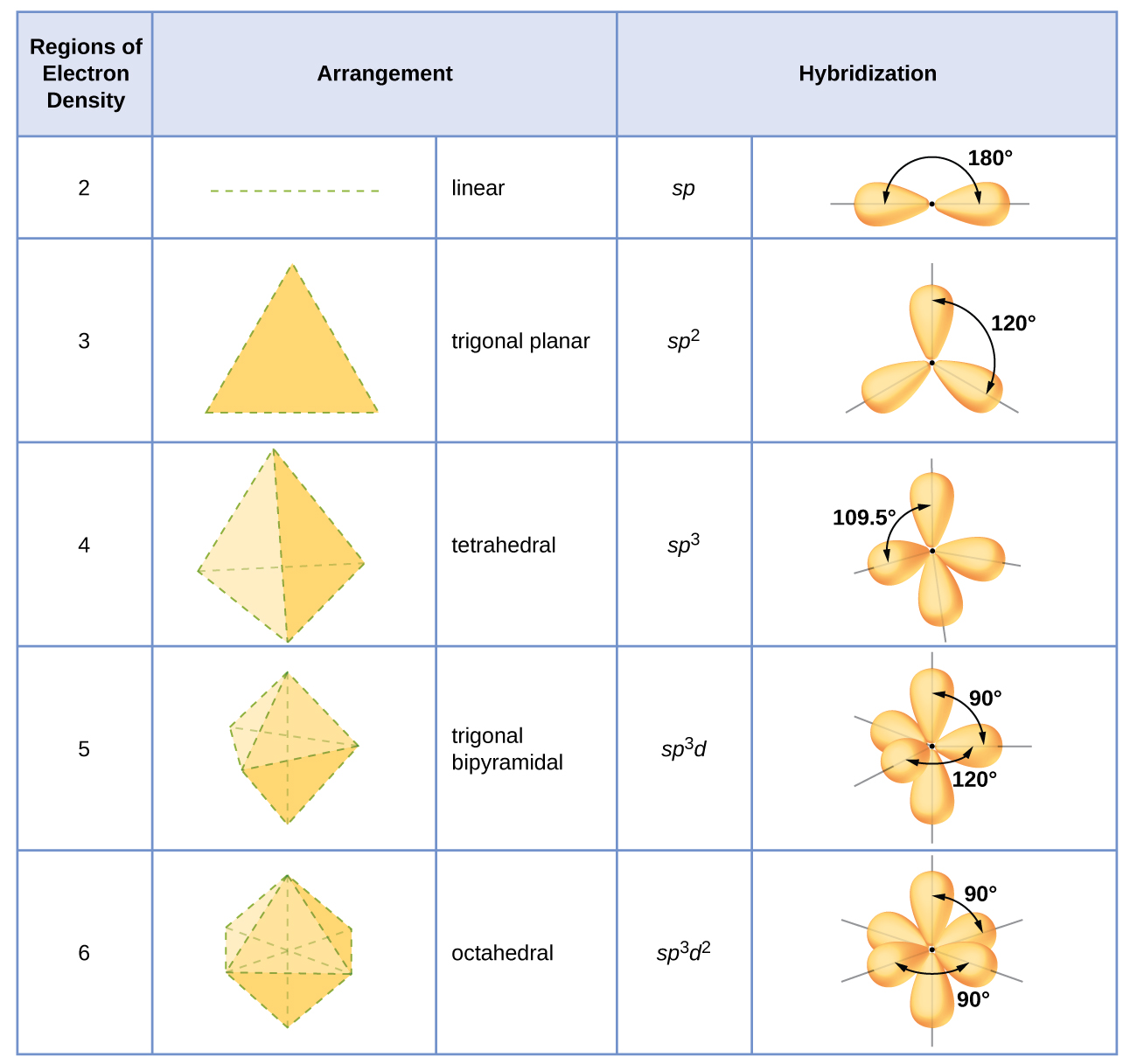
Bond angle 2 sp 180o 3 sp2 120o 4 sp3 1095o However there is an even easier way to judge the hybridization.

. Number of sigma bonds in the molecule. Determine the number of regions of electron density around an atom using VSEPR theory in which single bonds multiple bonds radicals and lone pairs each count as one region. Hybridization of CO 2.
Therefore it forms 3. It is better to write the Lewis structural formula to get a rough. 1 bond to another atom or lone pair s not really hybridized 2 bonds to another atom or lone pairs sp 3 bonds to another atom or lone pairs sp 2.
Method to find out Hybridization of the central atom of a molecule. Carbon can have an sp hybridization when it is bound to two other atoms with the help of two double bonds or one single and one triple bond. The number of sigma bonds is equal to the.
One triple bond sp. You can find the hybridization of an atom by finding its steric number. The following rules give the hybridization of the central atom.
To put it plain I can summarize the hybridizations in the following picture. How many electron domains areas of electrons are there around the central carbon. Power on the Hybridization state of the central atom Total no of σ bonds around each central atom -1 All single - bonds are σ bond in double bond there is one σ and 1π in triple bond there is one σ and 2π.
This is a simple way to find out the hybridization for an atom of carbon nitrogen or oxygen. The valency of nitrogen is 3. Another way to find out the hybridization of the molecule is to look at the number of sigma bonds formed.
5 EASY STEPS TO GET THE TYPE OF HYBRIDIZATION SHAPE. HOW TO FIND HYBRIDIZATION OF CENTRAL ATOM SHAPE OF MOLECULE. We can calculate the hybridization of OCl 2 using the steric number formula given below.
You can re-draw the bonds as pairs of Electrons - this might help show how there are 4. SIMPLE WAY to work out hybridization. If you look at the Nitrogen it has 4 different electron groups around it 3 from the bonds 1 from lone pair.
Physical Chemistry for the Biosciences. When the hybridization occurs the molecules have a linear arrangement of the atoms with a bond angle of 180. Determine the number of regions of electron density around an atom using VSEPR theory in which single bonds multiple bonds radicals and lone pairs each count as one region.
To find the hybridization of a central atom we can use the following guidelines. Hybridization happens only during the bond formation and not in an isolated gaseous atom. Determine the Lewis structure of the molecule.
To find the hybridization of a central atom we can use the following guidelines. For CH4 the hybridization is sp3. The steric number the number of atoms bonded to the atom the number of lone pairs the atom has.
A carbon atom is sp2 hybridized when. In other words you only have to count the number of bonds or lone pairs of electrons around a central atom to determine its hybridization. All single bonds or lone pairs sp 3.
In HCN there are two sigma bonds C-H and C-N. Determine the number of regions of electron density around an atom using VSEPR theory in which single bonds multiple bonds radicals and lone pairs each count as one region. The easiest way to determine hybridization is to with the VSEPR theory and determine the number of electron groups around your central atom.
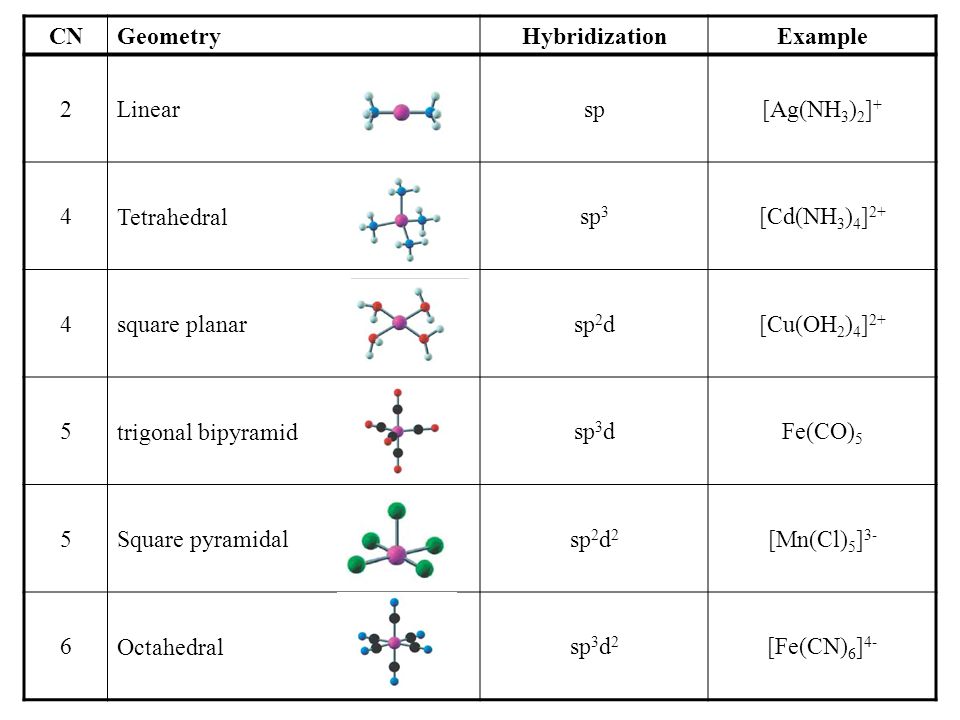
DETERMINING THE HYBRIDIZATION OF NITROGEN IN AMMONIA NH 3. The shape of the molecule can be predicted if the hybridization of the molecule is known. Molecule Hybridization Molecular Geometry Tetrahedral Sp3 CHCH acetylene H-CEC-H Trigonal Pyramidal Sp2 Trigonal Planar sp Linear s-only Bent p-only not hybridized sp3 Tetrahedral BCl3 boron trichloride CI Trigonal Pyramidal Sp2 Trigonal Planar sp Linear CI-B-Cl.
For NF_3 the Lewis Structure will give you something like Nitrogen in the center with 3 bonds to F atoms and 1 lone pairI dont know how to draw structures on here. One double bond sp 2. Formula used for the determination of sp sp2 and sp3 hybridization state.
Determine the Lewis structure of the molecule. Be sure to use this very helpful trick to help find the hybridization of an atom in a compoundPlease leave any comments questions and suggestions below. All single bonds or lone pairs sp3 One double bond sp2 One triple bond sp Chang Raymond.
4 and sp3 represents 4 hybridized orbitals. This is a simple way to find out the hybridization for an atom of carbon nitrogen or oxygen. If the steric number is 4 the atom is mathrmsp3 hybridized.
Circle the correct hybridization around the central atom and molecular geometry of the following molecules. For C2H2 acetylene the hybridization is sp representing 2 electron domains. Generally molecules with linear molecular geometry have sp hybridization as the central atom forms bonds with two atoms only.
The bigger lobe of the hybrid orbital always has a positive sign while the smaller lobe on the opposite side has a negative sign. Determine the Lewis structure of the molecule. To find the hybridization of a central atom we can use the following guidelines.
There is a cheesy-easy way to determine hybridization on central atoms.
8 2 Hybrid Atomic Orbitals Chemistry

Hybridization Of Atomic Orbitals Ck 12 Foundation
What Hybridization Is Generally Utilized By The Central Atom In A Square Planar Molecule Socratic

Question Video Identifying The Number Of Hybrid Orbitals Formed In Sp 𝑛 Hybridization Nagwa
0 Response to "How to Find Hybridization Around Central Atom"
Post a Comment